Navigation
 Home
Home Search the site
Search the site Project overview
Project overview The platform
The platform Medical background
Medical background Partners
Partners Advisory Boards
Advisory Boards Work plan
Work plan Deliverables
Deliverables Scientific papers
Scientific papers Downloads
Downloads Online demonstrators
Online demonstrators FAQ
FAQ All news
All news Reaction in the press
Reaction in the press Events
Events Clustering event
Clustering event Video archive
Video archive Relevant links
Relevant links Event Calendar
Event CalendarAffiliations
REACTION is affilliated with these programs and organisations: REACTION is delivering better and more efficient healthcare services in Europe, thus supporting the Commissions activities in ICT for Health: eHealth.
REACTION is delivering better and more efficient healthcare services in Europe, thus supporting the Commissions activities in ICT for Health: eHealth.
 The REACTION platform allows for the creation of inclusive applications with accessibility for all. The project supports the Commissions campaign: eInclusion - be part of it!
The REACTION platform allows for the creation of inclusive applications with accessibility for all. The project supports the Commissions campaign: eInclusion - be part of it!
Sign In

The REACTION project is a 4-year project started in 2010. It is partly funded by the European Commission under the 7th Framework Programme in the area of Personal Health Systems under Grant Agreement no. 248590
Newsletters
Read previous issues of our newsletter here:April 2014
August 2013
June 2012
August 2011
April 2011
November 2010
Most Read...
 In-hospital saf... In-hospital saf... |
187409 |
 Reaction in the... Reaction in the... |
116244 |
 Medical background Medical background |
110661 |
 Project overview Project overview |
107200 |
 Project methodo... Project methodo... |
81000 |
 Research award ... Research award ... |
58712 |
 IST-Africa Call... IST-Africa Call... |
58512 |
 REACTION at one... REACTION at one... |
56158 |
 REACTION videos... REACTION videos... |
51816 |
 REACTION online... REACTION online... |
49535 |
Popular Downloads
 REACTION brochure REACTION brochure |
30581 |
 Design of a mobil... Design of a mobil... |
13366 |
 D9-4 Healthcare e... D9-4 Healthcare e... |
11437 |
 D9-2 Regulatory f... D9-2 Regulatory f... |
10937 |
 2_Regulatory Fram... 2_Regulatory Fram... |
6836 |
Project methodology and workplan
REACTION is a 4-year research project with an iterative development approach.
The REACTION project seeks to use technology as a tool to foster Human Development, in order to use the great potential that new technologies might have for addressing major societal challenges in coping with the increasing number of citizens suffering from insulin-depending diabetes. The success of the new technological applications depends heavily on the acceptance from end-users, i.e. patients, relatives and professional carers as well as the acceptance from healthcare commissioners, business stakeholders, and regulatory authorities.
The requirement engineering process follows the principles of the ISO 13407 "Human-centred design processes for interactive systems" standard. This standard provides guidance on human-centred design activities throughout the life cycle of computer-based interactive systems. The REACTION project has adopted an evolutionary requirements engineering, specification and design methodology underpinned by a strong user-centric development, which comply with the following template in each iteration: " User requirements engineering and refinement " Architecture design specification and refinement " Clinical protocol and medical context planning " Technologies research and development to implement architecture " Integration and prototype development and field trial preparation " Field trials in clinical domains " Conformance testing, usability evaluation and user acceptance testing " Lessons Learned and change analysis.
Overview of the iterative approach
The methodology calls for comprehensive iterative requirements and stakeholder analysis based on initial requirements gathered from medical and clinical scenario thinking. These requirements would encompass the needs and priorities of the users as well as the wider exploitability and scalability requirements taking into account the technical constraints as well as the safety, socio-economic and legal acceptance and the deployability of the resulting REACTION platform in real Public Healthcare Systems.
The starting point of the iterative design process is a set of domain-specific vision scenarios delivering end-user visions of applications in three different insulin therapy domains: General Ward, Outpatient and Automatic Glucoses Control. The vision scenarios are used to derive detailed technical and clinical oriented use cases that will be discussed in focus groups with stakeholders. The result of this work will be an initial set of requirements specifications for the REACTION platform and applications. From the initial set of requirements, the software experts will specify the initial architectural specification. The architectural specification then drives the research and development work.
At the end of each iterative cycle (corresponding to one calendar year) a prototype of the REACTION platform will be implemented with a view to integrate as many as the existing components available at the time and in accordance with the detailed work plan. The clinical work will be undertaken in parallel to the technical development. It starts with the establishment of a set of clinical protocols in each of the three domains. The protocols define and drive clinical field trials, which will be performed using the increasingly advanced platform prototypes during the cause of the project. The field trials will be used for validation of the benefit provided for individual users and healthcare organisations in terms of efficiency of closed loop healthcare provisioning in diabetes management and insulin therapy. The socio-economic framework will be analysed with focus on ethics, legal and regulatory framework and the business eco-system for private and public stakeholders. The validation outcome together with results and requirements will be fed back to drive the requirements re-engineering work.
Overview of work plan
The project will be implemented with research and technological development being performed in twelve dedicated Work Packages (WPs) structured in a matrix organisation, as depicted in the figure below.
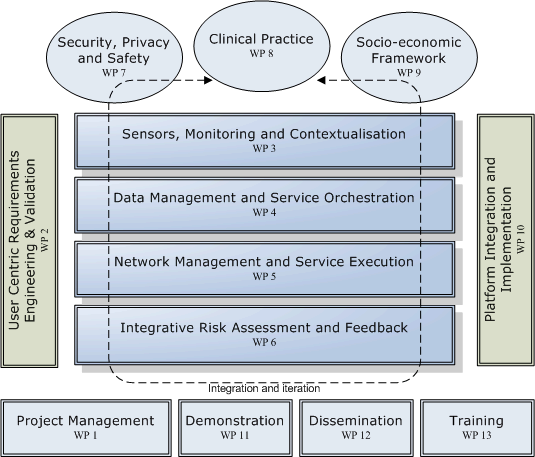
The technological research and development will be performed in packages (WP3-6) and security, privacy and safety (WP7). These activities will be supported and integrated with multidisciplinary research in clinical practice in diabetes management (WP8) and socio-economic frameworks (WP9). The work will follow an evolutionary requirement and usability engineering methodology managed from WP2 and implement and validate a total of four prototypes in three field trials. The activities and duration of the work packages are as follows:
![]() WP 1 Project Management
WP 1 Project Management
The workpackage will provide an effective and efficient management and work process of the project during the contractual period. Its objective is to ensure an efficient management of the project and a consistent high quality of the work to be performed and of the reports produced. The workpackage will be lead by the coordinator ATOS.
![]() WP 2 User Centric Requirements Engineering and Validation
WP 2 User Centric Requirements Engineering and Validation
The work in WP2 will manage and undertake the iterative engineering of requirements, which special focus on the engineering process of initial requirements and after the end of each iteration cycle. Lessons Learned obtained during project progress will be used to arrive at adjustments to the initial requirements incorporating and inclusion of emergent requirements. The workpackage will be lead by IN-JET.
![]() WP 3 Sensors, Monitoring and Contextualisation
WP 3 Sensors, Monitoring and Contextualisation
The first objective of this workpackage is to research and develop several types of non-invasive and minimally invasive wearable sensors for continuous monitoring of blood glucose levels.
The second objective is to investigate the requirements on measurement accuracy and applicability of several sensor technologies to be used in Automatic Glucose Control systems and optimise the sensor design for such system. The third objective is to develop a set of wearable and portable medical devices that can be coupled seamlessly with the REACTION platform for multi-parametric monitoring and contextualisation of health status. The workpackage will be lead by IMM.
![]() WP 4 Data Management and Service Orchestration
WP 4 Data Management and Service Orchestration
The objectives of this WP are to research, develop and implement the very complex Data Management structure of the REACTION platform. The WP will design and implement a semantic data management server that will provide the needed information management and service orchestration functionality. The workpackage will be lead by CNET.
![]() WP 5 Network Management and Service Execution
WP 5 Network Management and Service Execution
The main objective of this WP is to research, develop and implement the production platform on which REACTION applications can be executed. The first objective of this workpackage is to implement the network platform that allows seamless wireless connectivity and operability. The second objective is to develop the Network Management subset responsible for the physical communication between objects (devices, persons and external repositories). The third objective is to define and develop a resilient and intelligent event handling mechanism that can support crisis management and interface to established emergency centres. The workpackage will be lead by FORTHNET.
![]() WP 6 Integrative Risk Assessment and Feedback
WP 6 Integrative Risk Assessment and Feedback
The work in this workpackage will focus on developing integrative tools for analysis and correlation of the multi-parametric data with established biomedical knowledge and expertise to derive clinically relevant and useful information. It will develop strategies and tools for personalization of the disease management including the continuous control of insulin dosing that will be founded on evidence based knowledge for the patient population as well as individual anamnesis information and biomarker measurements. The workpackage will be lead by MSG.
![]() WP 7 Security, Privacy and Safety
WP 7 Security, Privacy and Safety
The objective of this workpackage is to develop a visible and controllable distributed security and privacy model, based on the concept of trust as a multilateral relation between stakeholders in a community of patients, informal carers and formal healthcare providers. The workpackage will be lead by FHG-SIT.
![]() WP 8 Clinical Practice and Field Trials
WP 8 Clinical Practice and Field Trials
The overall aim of this work package is to assess the effectiveness of the REACTION platform in three treatment scenarios addressed by the project, namely (i) within a hospital environment, (ii) outpatients under therapeutic control and for (iii) patients who are self-managing their disease. The goal is to conclusively prove the validity of the applications, demonstrate the benefit for healthcare providers and provisioning authorities, gain acceptance by patients and other users and to assess the impact at the organizational level. The workpackage will be lead by MUG.
![]() WP 9 Socio-economic Framework
WP 9 Socio-economic Framework
The objective of this workpackage is to investigate and provide overview of the ethical, social, legal, regulatory, and economic aspects of the REACTION platform in order to provide the best possible framework for the successful deployment of REACTION applications in the future. The workpackage will be lead by VUB.
![]() WP 10 Platform Implementation
WP 10 Platform Implementation
This WP deals with the integration of all the parts of the REACTION platform, the technical testing of its functionality and the setting up of prototypes to be used in the clinical field trials in WP8. The workpackage leader is FORTH-ICS.
![]() WP 11 Demonstration
WP 11 Demonstration
The demonstration activities are designed to prove the viability of the REACTION platform before it can be commercialised, e.g. testing of product-like prototypes. This type of activity goes beyond the validation activities internal to the project, which will be based on prototypes with limited functionality. The workpackage will be lead by CNET.
![]() WP 12 Dissemination and Exploitation
WP 12 Dissemination and Exploitation
The overall objective of this work package is to provide an active and professional dissemination of the project results according to the dissemination strategy described in section 3.2 Dissemination and/or exploitation of project results. The workpackage will be lead by IN-JET.
![]() WP 13 Training
WP 13 Training
The REACTION project will produce a set of professional course material in the form of websites, lecture notes and other printed and multimedia material. The first set of course materials to be produced will be targeted at healthcare solution developers. This material will be derived from the project internal technical training courses. The workpackage will be lead by UBRUN.
![]() Deliverables
Deliverables
The project will deliver a total of 105 deliverables over the four year period. Deliverables, which are dessignated as public, will be available for download here on the project's website. A full list of deliverables can be found here.


